Atomic Structure
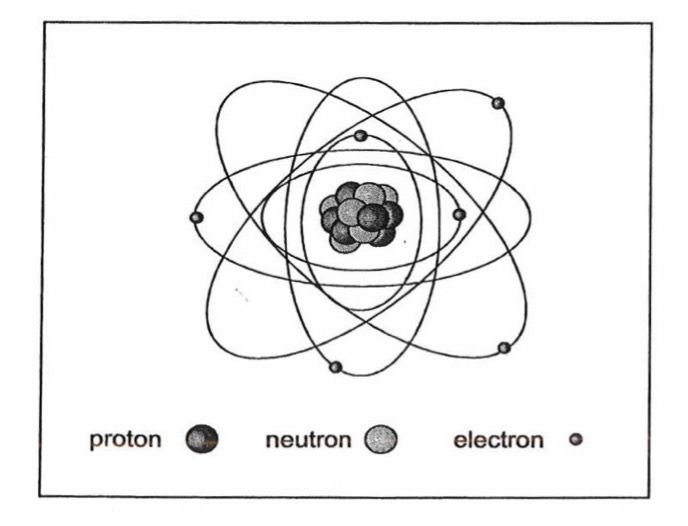
Iron has an atomic number of 26 which means that its atom contains 26 protons and 26 electrons there are also 30 neutrons in the core giving an atomic weight of 56.
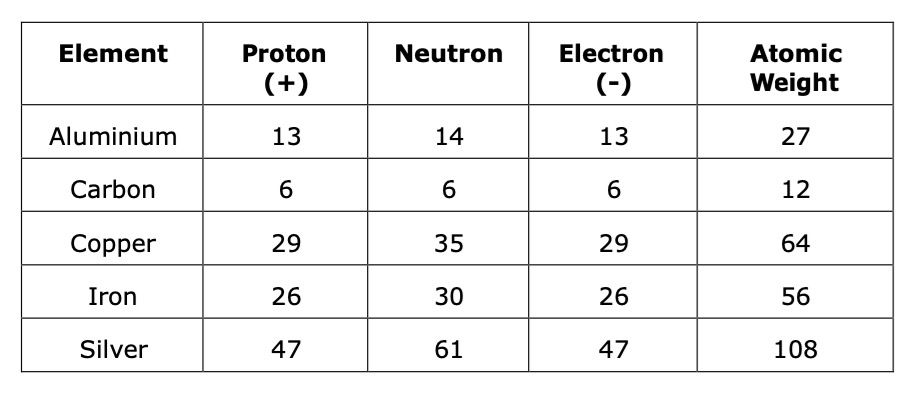
Protons have a positive charge and electrons a negative charge of equal but opposite value. The number of protons and electrons is the same, giving the atom a neutral charge. The nucleus of the atom also contains a number of neutrally charged particles called neutrons and the number of protons and neutrons gives the element its atomic weight.
The amount of metals present in the earths crust is variable, for instance, iron is 4.3%, aluminium 7.4%, copper 0.01%, silver 0.0001%. They are not, however, spread evenly so some areas are rich and some contain none.
The ores are mined at source and by stages separated from the unwanted material for refining. Different materials have different methods of refining, for the purposes of these notes we will follow the process which produces engineering steel, steel being an alloy of iron, carbon and other materials to tailor its required properties.